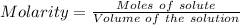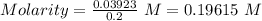Chemistry, 17.12.2019 04:31 simplydimps22owbohb
Asolution of nacl (aq) is added slowly to a solution of lead nitrate, pb(no3)2 ( aq ), until no further precipitation occurs. the precipitate is collected by filtration, dried, and weighed. a total of 10.91 g pbcl2 (s) is obtained from 200.0 ml of the original solution. calculate the molarity of the pb(no3)2 (aq) solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Asolution of nacl (aq) is added slowly to a solution of lead nitrate, pb(no3)2 ( aq ), until no furt...
Questions



Chemistry, 09.03.2020 05:50


History, 09.03.2020 05:51



Mathematics, 09.03.2020 05:51

Health, 09.03.2020 05:51

Mathematics, 09.03.2020 05:51

Business, 09.03.2020 05:51

Mathematics, 09.03.2020 05:52

Biology, 09.03.2020 05:52

Mathematics, 09.03.2020 05:52

Mathematics, 09.03.2020 05:52

Mathematics, 09.03.2020 05:53



Mathematics, 09.03.2020 05:53

Mathematics, 09.03.2020 05:53

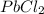 as:-
as:-
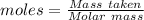
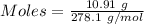
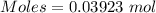
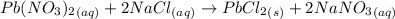
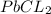 is produced when 1 mole of
is produced when 1 mole of 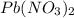 undergoes reaction.
undergoes reaction.