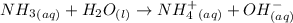Chemistry, 17.12.2019 04:31 brionnashelp
An aqueous solution of ammonia is found to be basic. this observation can be explained by the net ionic equationhno3(aq) + h2o(l) → no3-(aq) + h3o+(aq).nh4+(aq) + h2o(l) → nh3(aq) + h3o+(aq).no3-(aq) + h2o(l) → hno3(aq) + oh-(aq).nh3(aq) + h2o(l) → nh4+(aq) + oh-(aq).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
An aqueous solution of ammonia is found to be basic. this observation can be explained by the net io...
Questions








Spanish, 29.07.2019 20:30

Social Studies, 29.07.2019 20:30


Chemistry, 29.07.2019 20:30


Biology, 29.07.2019 20:30




Health, 29.07.2019 20:30