
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the presence of a suitable catalyst to form nitric oxide and water vapor: 4 nh31g2+5 o21g2¡4 no1g2+6 h2o1g2how many liters of nh31g2 at 850 °c and 5.00 atm are required to react with 1.00 mol of o21g2 in this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Nthe first step of the industrial process for making nitric acid, ammonia reacts with oxygen in the...
Questions

Medicine, 23.05.2021 06:20



Health, 23.05.2021 06:20

Physics, 23.05.2021 06:20


Biology, 23.05.2021 06:20


Mathematics, 23.05.2021 06:20



Chemistry, 23.05.2021 06:20


Mathematics, 23.05.2021 06:20


Mathematics, 23.05.2021 06:20

English, 23.05.2021 06:20

Mathematics, 23.05.2021 06:20

Mathematics, 23.05.2021 06:20

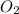 = 1.00 moles
= 1.00 moles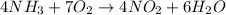
 moles of ammonia
moles of ammonia 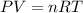
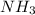 required.
required.

