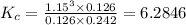Chemistry, 17.12.2019 05:31 Shadow0202
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperatures. ch₄ + h₂ ⇌ 3h₂ + what is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: ch₄, 0.126 m; h₂o, 0.242 m; co, 0.126 m; h₂ 1.15 m, at a temperature of 760 °c?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
You know the right answer?
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperature...
Questions





Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

History, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

English, 20.09.2020 16:01



Mathematics, 20.09.2020 16:01

English, 20.09.2020 16:01

English, 20.09.2020 16:01


Physics, 20.09.2020 16:01



Mathematics, 20.09.2020 16:01

![[CH_4]=0.126\ M](/tpl/images/0421/9784/de111.png)
![[H_2O]= 0.242\ M](/tpl/images/0421/9784/d80cb.png)
![[CO]= 0.126\ M](/tpl/images/0421/9784/495e0.png)
![[H_2]= 1.15\ M](/tpl/images/0421/9784/0632f.png)
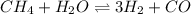
![K_{c}=\frac {\left [ H_2 \right ]^3\left [ CO \right ]}{\left [ CH_4 \right ]\left [ H_2O \right ]}](/tpl/images/0421/9784/6aeab.png)