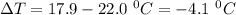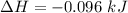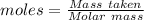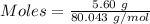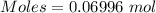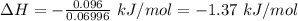Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 is dissolved in 100g of water at 22.0c; the temperature falls to 17.9c. assuming that the specific heat capacity of the solution is 4.18 j/(g*k), calculate the enthalpy of dissolution of nh4no3, in kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 i...
Questions







Biology, 16.02.2022 02:10




Mathematics, 16.02.2022 02:10





Mathematics, 16.02.2022 02:10



SAT, 16.02.2022 02:10

English, 16.02.2022 02:10

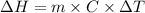
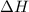 is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
is the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is the temperature change
is the temperature change