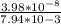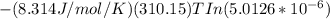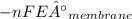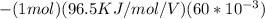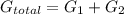Chemistry, 17.12.2019 06:31 rostecorralmart
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreting cells. the cells secrete hcl, proteolytic enzyme zymogens, mucin, and intrinsic factor. the ph of gastric juice is acidic, between 1-3. if the ph of gastric juice is 2.1, what is the amount of energy (? g) required for the transport of hydrogen ions from a cell (internal ph of 7.4) into the stomach lumen? assume that the potential difference across the membrane separating the cell and the interior of the stomach is �60.0 mv (inside of cells negative relative to the lumen of the stomach).
assume that the temperature is 37 �c.
the faraday constant is 96.5 kj�v�1�mol�1 and the gas constant is 8.314� 10�3 kj�mol�1�k�1. express your answer in kj/mol.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

You know the right answer?
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreti...
Questions

Mathematics, 27.11.2019 11:31

Social Studies, 27.11.2019 11:31

Chemistry, 27.11.2019 11:31


English, 27.11.2019 11:31



History, 27.11.2019 11:31


Mathematics, 27.11.2019 11:31

Mathematics, 27.11.2019 11:31

History, 27.11.2019 11:31

Advanced Placement (AP), 27.11.2019 11:31

English, 27.11.2019 11:31


Mathematics, 27.11.2019 11:31

English, 27.11.2019 11:31

History, 27.11.2019 11:31


Geography, 27.11.2019 11:31


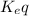 = equilibrum constant for the process
= equilibrum constant for the process
![\frac{[H^+]_(cell)}{[H^+(stomach lumen)]}](/tpl/images/0422/0463/00489.png)
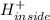 ⇒
⇒ 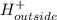
![K_{eq}=\frac{[H^+]_{outside}}{[H^+]_{inside}}](/tpl/images/0422/0463/bf29c.png)
![=\frac{[H^+]_{cell}}{[H^+]_{stomach lumen}}](/tpl/images/0422/0463/cc76d.png)
![[H^+]_{cell}](/tpl/images/0422/0463/36d6e.png) = 10⁻⁷⁴
= 10⁻⁷⁴ ![[H^+]_{stomach lumen}](/tpl/images/0422/0463/f5443.png) = 10⁻²¹
= 10⁻²¹![K_{eq}=\frac{[H^+]_{cell}}{[H^+]_{stomachlumen}}](/tpl/images/0422/0463/c2f36.png)