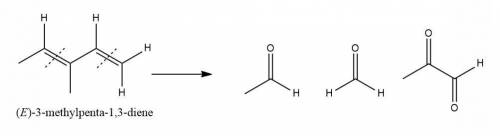
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went from orangish/red to clear immediately at room temperature. upon treatment with o3 followed by work-up with dimethylsulfide the following products were identified. from the information provided what is/are the most likely structure(s) for this unknown compound.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
An unknown compound with empirical formula c3h5 was treated with br2/ccl4. the bromine solution went...
Questions




Spanish, 31.03.2020 22:31

History, 31.03.2020 22:31

Mathematics, 31.03.2020 22:32

Mathematics, 31.03.2020 22:32



Mathematics, 31.03.2020 22:32

Social Studies, 31.03.2020 22:32

Mathematics, 31.03.2020 22:32


Mathematics, 31.03.2020 22:32

Mathematics, 31.03.2020 22:32

Mathematics, 31.03.2020 22:32

Mathematics, 31.03.2020 22:32


History, 31.03.2020 22:32

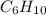 .
.