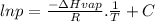Chemistry, 17.12.2019 21:31 lopeznadia838
The vapor pressure of a substance is measured over a range of temperatures. a plot of the natural log of the vapor pressure versus the inverse of the temperature (in kelvin) produces a straight line with a slope of −3.58×103k. what is the enthalpy of vaporization of the substance? 29.8 kj/mol2.32×1023kj/mol0.431 kj/mol294 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
The vapor pressure of a substance is measured over a range of temperatures. a plot of the natural lo...
Questions

Mathematics, 25.02.2021 19:20

Chemistry, 25.02.2021 19:20

History, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20

English, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20

History, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20



English, 25.02.2021 19:20

Engineering, 25.02.2021 19:20

Chemistry, 25.02.2021 19:20



English, 25.02.2021 19:20

Mathematics, 25.02.2021 19:20


Mathematics, 25.02.2021 19:20

English, 25.02.2021 19:20