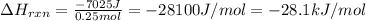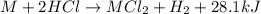Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted wit...

Chemistry, 17.12.2019 21:31 colestout2993
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 7025 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? )

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Questions




Social Studies, 01.01.2020 04:31


Mathematics, 01.01.2020 04:31

History, 01.01.2020 04:31





Social Studies, 01.01.2020 04:31


Mathematics, 01.01.2020 04:31

English, 01.01.2020 04:31

Mathematics, 01.01.2020 04:31


Mathematics, 01.01.2020 04:31

Mathematics, 01.01.2020 04:31

Mathematics, 01.01.2020 04:31

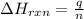
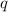 = amount of heat released = -7025 J
= amount of heat released = -7025 J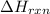 = enthalpy change of the reaction
= enthalpy change of the reaction