Using vsepr theory, which of these molecules does not have a trigonal planar shape?
a) ammoni...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Questions

Chemistry, 03.11.2020 18:40



Mathematics, 03.11.2020 18:40






Chemistry, 03.11.2020 18:40




History, 03.11.2020 18:40

Mathematics, 03.11.2020 18:40

Mathematics, 03.11.2020 18:40


Mathematics, 03.11.2020 18:40


Mathematics, 03.11.2020 18:40

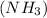 has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide
has 3 sigma bonds and one lone pair.Hence its shape is pyramidal due to bond pair - lone pair repulsion.Formaldehyde (HCHO) has 3 sigma bonds and 1 pi bond.Hence it has trigonal planar shape.Sulfur trioxide 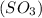 has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.
has 3 sigma bonds and 3 pi bonds.Boron triflouride (BF_3) has 3 sigma bonds.

