
Chemistry, 17.12.2019 22:31 missy922527
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) + cl2(g) pcl5(g) when 0.17 moles of cl2(g) are added to the equilibrium system at constant temperature: the value of kc the value of qc kc. the reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. it is already at equilibrium. the concentration of pcl3 will

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) +...
Questions











Computers and Technology, 27.06.2020 05:01

Health, 27.06.2020 05:01


Mathematics, 27.06.2020 05:01






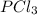 will decrease.
will decrease.

