
Chemistry, 17.12.2019 23:31 cathydaves
Carbon monoxide reacts with oxygen gas to form carbon dioxide according to the following chemical equation. 2co(g)+o2(g)⟶2co2(g) how many ml of oxygen gas will remain in the reaction vessel when 82.4ml of carbon monoxide gas reacts with 80.0ml of oxygen gas to form carbon dioxide under constant pressure and temperature?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2


Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Carbon monoxide reacts with oxygen gas to form carbon dioxide according to the following chemical eq...
Questions

Mathematics, 08.11.2020 09:30

Spanish, 08.11.2020 09:30

Spanish, 08.11.2020 09:30


Chemistry, 08.11.2020 09:30

Arts, 08.11.2020 09:30



Chemistry, 08.11.2020 09:40

English, 08.11.2020 09:40

Biology, 08.11.2020 09:40

Geography, 08.11.2020 09:40

History, 08.11.2020 09:40


English, 08.11.2020 09:40

History, 08.11.2020 09:40

Chemistry, 08.11.2020 09:40


English, 08.11.2020 09:40

Social Studies, 08.11.2020 09:40

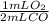 = 41.2 mL O₂
= 41.2 mL O₂

