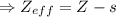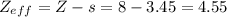Chemistry, 17.12.2019 23:31 Solany6527
Effective nuclear charge, zeff, is defined as:
zeff=z−s
where z is true nuclear charge and s is the amount of shielding.
in 1930, john c. slater devised the following set of empirical rules to estimate s for a designated ns or np electron:
1. write the electron configuration of the element, and group the subshells as follows: (1s), (2s, 2p), (3s, 3p), (3d), (4s, 4p), (4d), (4f ), (5s, 5p), and so on.
2. electrons in groups to the right of the (ns, np) group contribute nothing to the shielding constant for the designated electron.
3. all the other electrons in the (ns, np) group shield the designated electron to the extent of 0.35 each.
4. all electrons in the n−1 shell shield to the extent of 0.85 each.
5. all electrons in the n−2 shell, or lower, shield completely—their contributions to the shielding constant are 1.00 each.
when the designated electron is in an nd or nf group, rules (i), (ii), and (iii) remain the same but rules (iv) and (v) are replaced by the following: each electron in a group lying to the left of the nd or nf group contributes 1.00 to the shielding constant. these rules are a simplified generalization based on the average behavior of different types of electrons.
part a) calculate zeff for a valence electron in an oxygen atom.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Effective nuclear charge, zeff, is defined as:
zeff=z−s
where z is true nuclear charg...
zeff=z−s
where z is true nuclear charg...
Questions

Spanish, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

English, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

Chemistry, 22.10.2020 18:01

Chemistry, 22.10.2020 18:01



Chemistry, 22.10.2020 18:01

History, 22.10.2020 18:01

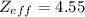
![[Z_{eff}]](/tpl/images/0423/1699/163e1.png) is the net nuclear charge experienced by the electron in a given atom. It is always less than the actual charge of the nucleus [Z], due to shielding by electrons in the inner shells.
is the net nuclear charge experienced by the electron in a given atom. It is always less than the actual charge of the nucleus [Z], due to shielding by electrons in the inner shells.