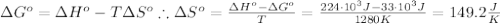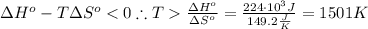The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and is approximately constant from 920 k up to 1280 k. the standard reaction free energy is +33 kj at 1280 k. calculate the equilibrium constant at 1280 k and then calculate the temperature at which the equilibrium constant becomes greater than 1.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
The standard reaction enthalpy of zn(s) + h2o(g) →zno(s) + h2(g) is known to be hr 0 = 224 kj and i...
Questions

Physics, 22.09.2019 14:00



Mathematics, 22.09.2019 14:00

History, 22.09.2019 14:00



Health, 22.09.2019 14:00

Mathematics, 22.09.2019 14:00

History, 22.09.2019 14:00



Mathematics, 22.09.2019 14:00

Biology, 22.09.2019 14:00

Geography, 22.09.2019 14:00




Mathematics, 22.09.2019 14:00

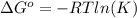
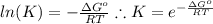
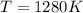 and the ideal gas law constant
and the ideal gas law constant 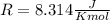 , we obtain:
, we obtain: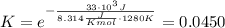
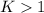 , then
, then 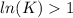 and
and 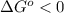 .
.