
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the change in energy according to the law of conservation of energy.
a) there is a net gain of 185 kj of energy when decomposing hcl.
b) it takes more energy to break the bonds of hcl than form h2(g) + cl2(g).
c) the energy required to decompose hcl and produce h2(g) + cl2(g) are equal.
d) it takes less energy to break the bonds of hcl than form the bonds of h2(g) + cl2(g.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1
You know the right answer?
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
Questions


Mathematics, 29.03.2021 19:20


History, 29.03.2021 19:20





Mathematics, 29.03.2021 19:20


History, 29.03.2021 19:20

Mathematics, 29.03.2021 19:20

English, 29.03.2021 19:20







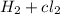 are equal.
are equal.

