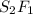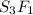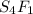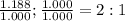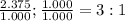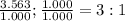3. sulfur (s) and fluorine (f) combine to form three different compounds. compound ahas 1.118 g of f for every 1.000 9 s. compound bhas 2.375 g of f
for every 1.000 9 s. compound chas 3.563 of f for every 1.000 g s. how do these compounds support the law of multiple proportions?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
3. sulfur (s) and fluorine (f) combine to form three different compounds. compound ahas 1.118 g of f...
Questions

History, 31.01.2020 21:49

Mathematics, 31.01.2020 21:49

History, 31.01.2020 21:49

Mathematics, 31.01.2020 21:49

Mathematics, 31.01.2020 21:49

History, 31.01.2020 21:49


Mathematics, 31.01.2020 21:49









English, 31.01.2020 21:49

Mathematics, 31.01.2020 21:49

Mathematics, 31.01.2020 21:49

Business, 31.01.2020 21:49