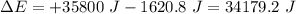Chemistry, 18.12.2019 02:31 gwendallinesikes
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is expanding from 8.00 to 24.0 l in volume at 1.00 atm. (remember that 101.3 j = 1 l·atm)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2


Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is e...
Questions

English, 09.04.2021 02:50

Mathematics, 09.04.2021 02:50



English, 09.04.2021 02:50

Mathematics, 09.04.2021 02:50

Social Studies, 09.04.2021 02:50


Mathematics, 09.04.2021 02:50



Social Studies, 09.04.2021 02:50



Computers and Technology, 09.04.2021 02:50

Mathematics, 09.04.2021 02:50

Arts, 09.04.2021 02:50

Mathematics, 09.04.2021 02:50


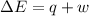
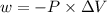
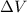 is the change in volume
is the change in volume
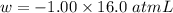
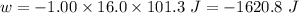 (work is done by the system)
(work is done by the system)