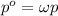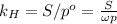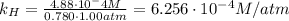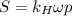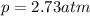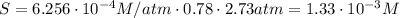Chemistry, 18.12.2019 04:31 terrysizemore666
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure and 25.0 degreec, the n2 component will dissolve in water with a solubility of 4.88 x 10^-4m. what is the value of henry's law constant for n2 under these conditions? express the constant numerically inmoles per liter per atmosphere. =6.26? 10^-4 mol/(l. atm) correct part b as a scuba diver descends under water, thepressure increases. at a total air pressure of 2.73 atm and a temperature of 25.0 degreec, what is the solubility of n2 in a diver's blood? [use the value of the henry's lawconstant calculated in part a, 6.26 x 10^-4mol/(l. atm). assume that the composition of the air in the tank is the sameas on land and that all of the dissolved nitrogen remains in theblood. express your answer numerically inmoles per liter. solubility =mol/l

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Air is a mixture of gases that is about78.0percent n2 by volume. when air is at standard pressure an...
Questions

Mathematics, 20.03.2021 14:00

Mathematics, 20.03.2021 14:00

Advanced Placement (AP), 20.03.2021 14:00

Mathematics, 20.03.2021 14:00


Mathematics, 20.03.2021 14:00


Social Studies, 20.03.2021 14:00





Mathematics, 20.03.2021 14:00

Mathematics, 20.03.2021 14:00

English, 20.03.2021 14:00


Mathematics, 20.03.2021 14:00

Mathematics, 20.03.2021 14:00


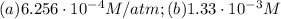
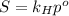
![p = 1.00 atm[/atm]Nitrogen's pecentage is:[tex]\omega = 0.780](/tpl/images/0423/6471/ab47c.png)