Chemistry, 18.12.2019 04:31 angelvega2003
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doing a famous experiment in 1775. in this experiment lavoisier found that mercury(ii) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
1. write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(ii) oxide (hgo) into liquid mercury and gaseous dioxygen.
2. suppose 50.0ml of dioxygen gas are produced by this reaction, at a temperature of 90°c and pressure of exactly 1atm. calculate the mass of mercury(ii) oxide that must have reacted. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
The great french chemist antoine lavoisier discovered the law of conservation of mass in part by doi...
Questions



History, 22.12.2021 09:00


Mathematics, 22.12.2021 09:00

SAT, 22.12.2021 09:00

SAT, 22.12.2021 09:00


Chemistry, 22.12.2021 09:00




Social Studies, 22.12.2021 09:00


Advanced Placement (AP), 22.12.2021 09:10





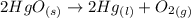
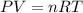
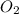 = 0.001677 mol
= 0.001677 mol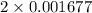 moles of mercury(II) oxide are reacted
moles of mercury(II) oxide are reacted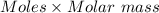 =
=