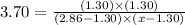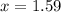For the chemical equation so 2 ( g ) + no 2 ( g ) − ⇀ ↽ − so 3 ( g ) + no ( g ) the equilibrium constant at a certain temperature is 3.70 . at this temperature, calculate the number of moles of no 2 ( g ) that must be added to 2.86 mol so 2 ( g ) in order to form 1.30 mol so 3 ( g ) at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
For the chemical equation so 2 ( g ) + no 2 ( g ) − ⇀ ↽ − so 3 ( g ) + no ( g ) the equilibrium cons...
Questions



Mathematics, 22.02.2021 07:50

Mathematics, 22.02.2021 07:50



Social Studies, 22.02.2021 07:50



Chemistry, 22.02.2021 07:50


Mathematics, 22.02.2021 08:00


Biology, 22.02.2021 08:00

English, 22.02.2021 08:00

Chemistry, 22.02.2021 08:00

Spanish, 22.02.2021 08:00



English, 22.02.2021 08:00

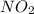 gas added must be, 1.59 moles.
gas added must be, 1.59 moles.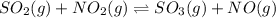
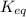 will be,
will be,![K_{eq}=\frac{[SO_3][NO]}{[SO_2]NO_2]}](/tpl/images/0423/7838/755be.png)