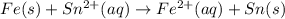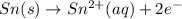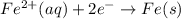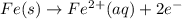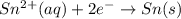What we call "tin cans" are really iron cans coated with a thin layer of tin. the anode is a bar of tin and the cathode is the iron can. an electrical current is used to oxidize the sn to sn2+ in solution, which is reduced to produce a thin coating of sn on the can.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
What we call "tin cans" are really iron cans coated with a thin layer of tin. the anode is a bar of...
Questions



Computers and Technology, 24.03.2021 20:30







Social Studies, 24.03.2021 20:30

Mathematics, 24.03.2021 20:30

Mathematics, 24.03.2021 20:30

Mathematics, 24.03.2021 20:30


Biology, 24.03.2021 20:30

English, 24.03.2021 20:30


History, 24.03.2021 20:30

Mathematics, 24.03.2021 20:30