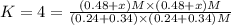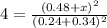Chemistry, 18.12.2019 05:31 djmelodiedaniels
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium concentrations are found to be [co2] = 0.24 m, [h2] = 0.24 m, [h2o] = 0.48 m, and [co] = 0.48 m. then an additional 0.34 moles per liter of co2 and h2 are added. when the reaction comes to equilibrium again at the same temperature, what will be the molar concentration of co?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium conce...
Questions

Mathematics, 01.12.2019 15:31

Computers and Technology, 01.12.2019 15:31

History, 01.12.2019 15:31

Mathematics, 01.12.2019 15:31

Biology, 01.12.2019 15:31

English, 01.12.2019 15:31

English, 01.12.2019 15:31










Mathematics, 01.12.2019 15:31

Mathematics, 01.12.2019 15:31

Mathematics, 01.12.2019 15:31

![[CO_2] = 0.24 M, [H_2] = 0.24 M, [H_2O] = 0.48 M, [CO] = 0.48 M](/tpl/images/0423/7365/45c13.png)
![K=\frac{[H_2O][CO]}{[CO_2][H_2]}=\frac{0.48 M\times 0.48 M}{0.24 M\times 0.24 M}](/tpl/images/0423/7365/2a444.png)

 and
and 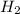 are added.
are added.