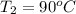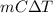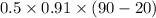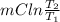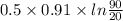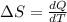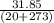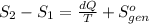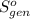Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. calculate (a) the change in stored energy (δe), (b) the amount of heat transfer (q), (c) the change in entropy (δs), (d) the amount of entropy transfer by heat and (e) the entropy generation (sgen, univ) in the system's universe during the heat transfer process.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

You know the right answer?
Ablock of aluminum with m = 0.5 kg, t = 20oc is dropped into a reservoir at a temperature of 90oc. c...
Questions

Mathematics, 19.11.2020 20:40

Mathematics, 19.11.2020 20:40


Mathematics, 19.11.2020 20:40

Mathematics, 19.11.2020 20:40

History, 19.11.2020 20:40

Chemistry, 19.11.2020 20:40



Mathematics, 19.11.2020 20:40



Mathematics, 19.11.2020 20:40





Arts, 19.11.2020 20:40

Mathematics, 19.11.2020 20:40

 ,
,