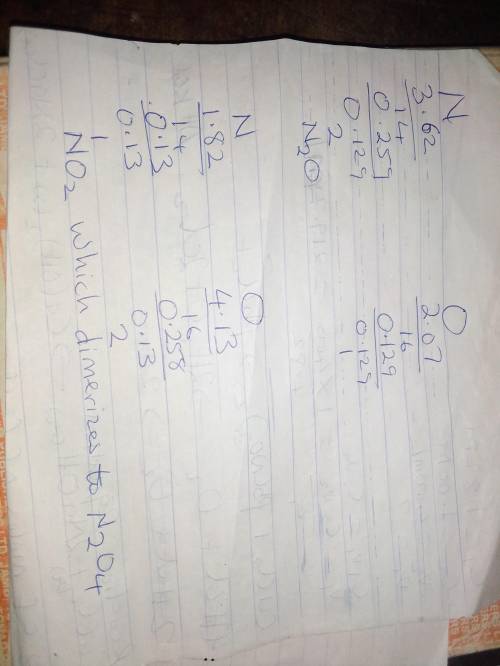Chemistry, 18.12.2019 05:31 alishajade
Nitrogen and oxygen can react to form various compounds.
two experiments showed that one compound is formed when 3.62 g of nitrogen and 2.07 g of oxygen react completely, while another compound is formed when 1.82 g of nitrogen reacts completely with 4.13 g of oxygen.
which of the following are most likely the molecular formulas for the nitrogen oxides obtained in these experiments? (1) no, n2o(2) no, no2(3) n2o, n2o5(4) no, n2o4(5) n2o, n2o4

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Nitrogen and oxygen can react to form various compounds.
two experiments showed that one comp...
two experiments showed that one comp...
Questions










Chemistry, 21.06.2019 21:00







History, 21.06.2019 21:00