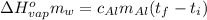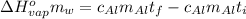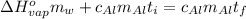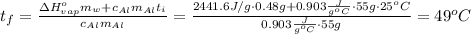Chemistry, 18.12.2019 06:31 hiiliohi3823
Suppose that 0.48 g of water at 25 ∘ c condenses on the surface of a 55- g block of aluminum that is initially at 25 ∘ c . if the heat released during condensation goes only toward heating the metal, what is the final temperature (in degrees celsius) of the metal block? (the specific heat capacity of aluminum, c s, al , is 0.903 j/(g⋅ ∘ c) .) express the temperature in degrees celsius to two significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Suppose that 0.48 g of water at 25 ∘ c condenses on the surface of a 55- g block of aluminum that is...
Questions


Mathematics, 22.07.2020 04:01




Mathematics, 22.07.2020 04:01














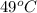
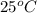 , the heat of vaporization of water is given by:
, the heat of vaporization of water is given by: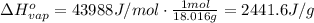
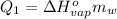
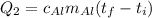
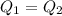 or:
or: