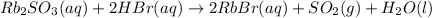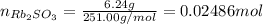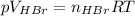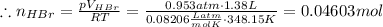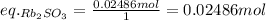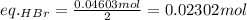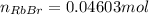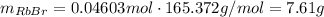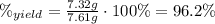Chemistry, 18.12.2019 07:31 Zachary429
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 atm pressure. the solid rbbr, extracted from the reaction mixture and purified, has a mass of 7.32 g.
(a) what is the limiting reactant?
(b) what is the theoretical yield of rbbr, assuming com- plete reaction?
(c) what is the actual percentage yield of product?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
Asolid sample of rb2so3 weighing 6.24 g reacts with 1.38 l gaseous hbr, measured at 75°c and 0.953 a...
Questions

Social Studies, 01.05.2021 01:00



Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00





Mathematics, 01.05.2021 01:00

Social Studies, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

History, 01.05.2021 01:00



Biology, 01.05.2021 01:00