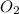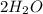Chemistry, 18.12.2019 08:31 starbae1084
Which statement best describes balancing equations and the law of conservation of mass?
there are more atoms in the reactants than in the products, and the total mass is the same in the reactants and in the
products
the number of atoms is the same in the reactants and in the products, and the total mass is the same in the reactants and
in the products,
there are fewer atoms in the reactants than in the products, and the total mass is less in the reactants than in the
products
there are more atoms in the reactants than in the products, and the total mass is higher in the reactants than in the
products
save and exit
next
mark this and return
submit

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Which statement best describes balancing equations and the law of conservation of mass?
there...
there...
Questions

Social Studies, 20.04.2021 21:20



Mathematics, 20.04.2021 21:20

Mathematics, 20.04.2021 21:20

Mathematics, 20.04.2021 21:20


Chemistry, 20.04.2021 21:20

History, 20.04.2021 21:20

History, 20.04.2021 21:20

English, 20.04.2021 21:20

Mathematics, 20.04.2021 21:20

English, 20.04.2021 21:20



Computers and Technology, 20.04.2021 21:20


Chemistry, 20.04.2021 21:20

History, 20.04.2021 21:20

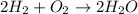
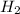 and
and