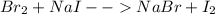Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai, respectively. when hexane was added to the test tubes, an orange color developed in the top layer of the first test tube, while purple developed in the second.
a. identify x2.
b. write a balanced equation for each reaction which occurred.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai,...
Questions


Mathematics, 16.10.2019 01:10





Mathematics, 16.10.2019 01:10












Mathematics, 16.10.2019 01:10

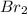
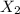 is
is 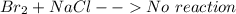 (because bromine is less reactive than chlorine)
(because bromine is less reactive than chlorine)