
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxygen gas to produce iron (iii) oxide. your sample of iron is 12.0 moles of iron. so which if these is a true statement? note: all numbers located immediately after elemental symbols below should be considered subscripts. a. 4.5 moles of o2 and produce 3.0 moles of fe2o3. b. 12.0 moles of o2 and produce 24.0 moles of fe2o3. c. 9.0 moles of o2 and produce 3.0 moles of fe2o3. d. 9.0 moles of o2 and produce 6.0 moles of fe2o3 e. none of the above

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

You know the right answer?
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxy...
Questions



History, 25.10.2019 19:43




Mathematics, 25.10.2019 19:43


History, 25.10.2019 19:43









Computers and Technology, 25.10.2019 19:43


English, 25.10.2019 19:43

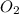 and produce 6.0 moles of
and produce 6.0 moles of 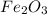
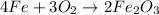
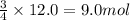 of oxygen gas
of oxygen gas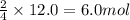 of iron (III) oxide
of iron (III) oxide

