Chemistry, 18.12.2019 23:31 4300402428
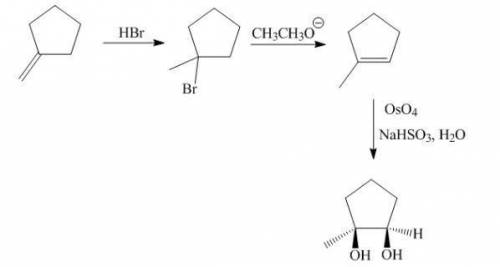
The target diol is synthesized in one step from 1-methylcyclopentene, but your lab partner exhausted the supply of that alkene. fortunately, you have plenty of isomers (c6h10) on hand from which to synthesize 1-methylcyclopentene and, ultimately, the diol. provide the missing reagents and organic structures needed to most efficiently produce the target product.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
The target diol is synthesized in one step from 1-methylcyclopentene, but your lab partner exhausted...
Questions

Mathematics, 28.10.2019 18:31

Biology, 28.10.2019 18:31


Social Studies, 28.10.2019 18:31




History, 28.10.2019 18:31



Mathematics, 28.10.2019 18:31

Geography, 28.10.2019 18:31

Biology, 28.10.2019 18:31


History, 28.10.2019 18:31





Engineering, 28.10.2019 18:31

 .
.