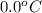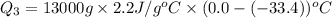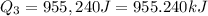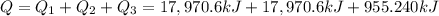Liquid ammonia, , was used as a refrigerant fluid before the discovery of the chlorofluorocarbons and is still widely used today. its normal boiling point is –33.4 °c, and its vaporization enthalpy is 23.5 kj/mol. the gas and liquid have specific heat capacities of 2.2 and 4.7 , respectively. calculate the heat energy transfer required to raise the temperature of 13.0 kg liquid ammonia from –50.0 °c to 0.0 °c. heat energy = kj

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

You know the right answer?
Liquid ammonia, , was used as a refrigerant fluid before the discovery of the chlorofluorocarbons an...
Questions

Biology, 22.01.2021 06:50

Mathematics, 22.01.2021 06:50

English, 22.01.2021 06:50


History, 22.01.2021 06:50

Mathematics, 22.01.2021 06:50

World Languages, 22.01.2021 06:50

Mathematics, 22.01.2021 06:50


Physics, 22.01.2021 06:50

Mathematics, 22.01.2021 06:50




Computers and Technology, 22.01.2021 06:50

Chemistry, 22.01.2021 06:50



Computers and Technology, 22.01.2021 06:50


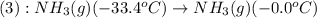

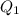 = amount of heat absorbed = ?
= amount of heat absorbed = ?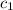 = specific heat of liquid ammonia =
= specific heat of liquid ammonia = 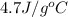
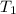 = initial temperature =
= initial temperature = 
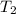 = final temperature =
= final temperature = 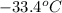
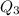 , we get:
, we get: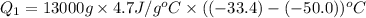
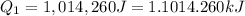
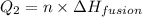
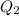 = amount of heat absorbed = ?
= amount of heat absorbed = ?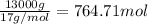
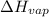 = enthalpy change for vaporization=23.5 kJ/mol
= enthalpy change for vaporization=23.5 kJ/mol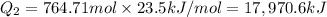
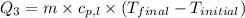
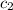 = specific heat of gaseous ammonia =
= specific heat of gaseous ammonia =