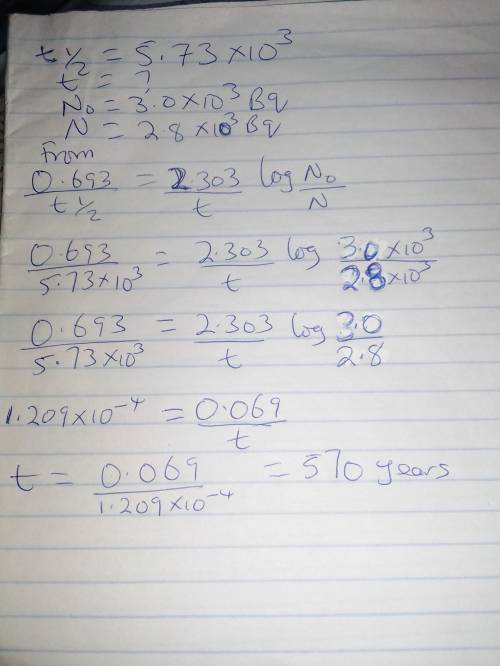Chemistry, 19.12.2019 00:31 Svetakotok
The half-life for the decay of carbon-14 is 5.73x10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of woodfrom an archeological dig is measured to be 2.8x10^3 bq. the activity in a similiar-sized sample of fresh wood is measured to be 3.0x10^3 bq. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
The half-life for the decay of carbon-14 is 5.73x10^3 years. suppose the activity due to the radioac...
Questions





Physics, 19.11.2020 18:40


Mathematics, 19.11.2020 18:40




Mathematics, 19.11.2020 18:40





Mathematics, 19.11.2020 18:40

Biology, 19.11.2020 18:40