
Chemistry, 19.12.2019 01:31 ayoismeisalex
The average bond energy (enthalpy) for a c=c double bond is 614 kj/mol and that of a c−c single bond is 348 kj/mol. estimate the energy needed to break only the π bond of the double bond of 2-butene. express your answer numerically in joules per molecule.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
The average bond energy (enthalpy) for a c=c double bond is 614 kj/mol and that of a c−c single bond...
Questions



Mathematics, 11.10.2019 08:10





Mathematics, 11.10.2019 08:10


Mathematics, 11.10.2019 08:10

Mathematics, 11.10.2019 08:10

Mathematics, 11.10.2019 08:10

Chemistry, 11.10.2019 08:10



Mathematics, 11.10.2019 08:10

History, 11.10.2019 08:10



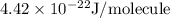 .
. 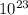
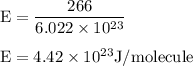
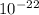 J/molecule.
J/molecule. 

