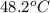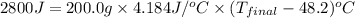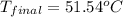Chemistry, 19.12.2019 02:31 mlarsen5000
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0 g solution at an initial temperature of 48.2°c. if the enthalpy of neutralization for the reaction between a strong acid and a strong base is −56 kj/mol, calculate the final temperature of the calorimeter contents. assume the specific heat capacity of the solution is 4.184 j°c⁻¹ g⁻¹ and assume no heat loss to the surroundings.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 150.0 ml of 0.50 m hcl is added to 50.0 ml of 1.00 m naoh to make 200.0...
Questions

Mathematics, 24.02.2021 01:00



Mathematics, 24.02.2021 01:00




Mathematics, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00



Mathematics, 24.02.2021 01:00



English, 24.02.2021 01:00


History, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

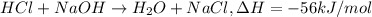
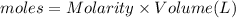
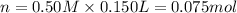
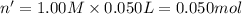
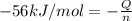
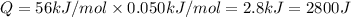
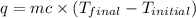
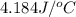
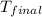 = final temperature =
= final temperature = 
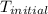 = initial temperature =
= initial temperature =