
Nitrogen (n2) enters a well-insulated diffuser operating at steady state at 0.656 bar, 300 k with a velocity of 282 m/s. the inlet area is 4.8 x 10-3 m2. at the diffuser exit, the pressure is 0.9 bar and the velocity is 140 m/s. the nitrogen behaves as an ideal gas with k = 1.4. (a) determine the exit temperature, in k, and the exit area, in m2. (b) for a control volume enclosing the diffuser, determine the rate of entropy production, in kw/k.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Nitrogen (n2) enters a well-insulated diffuser operating at steady state at 0.656 bar, 300 k with a...
Questions

Arts, 19.11.2020 23:10



Mathematics, 19.11.2020 23:10

English, 19.11.2020 23:10



Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

World Languages, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10


History, 19.11.2020 23:10



Social Studies, 19.11.2020 23:10

History, 19.11.2020 23:10

English, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

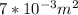 .The rate of entropy production would be 0.
.The rate of entropy production would be 0.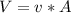 , Then for the inlet of the diffuser:
, Then for the inlet of the diffuser: 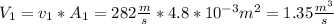 For an ideal gas working in an isentropic process, it follows that:
For an ideal gas working in an isentropic process, it follows that: 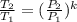 where each variable is defined according with what was presented in step 2 and 3, and k is the heat values relationship, 1.4 for nitrogen.Then solving for
where each variable is defined according with what was presented in step 2 and 3, and k is the heat values relationship, 1.4 for nitrogen.Then solving for 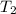 , the temperature of the nitrogen at the exit conditions:
, the temperature of the nitrogen at the exit conditions: 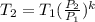 then,
then, 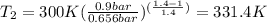 Also, for an ideal gas working in an isentropic process, it follows that:
Also, for an ideal gas working in an isentropic process, it follows that: 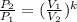 , where each variable is defined according with what was presented in step 2 and 3, and k is the heat values relationship, 1.4 for nitrogen.Then solving for
, where each variable is defined according with what was presented in step 2 and 3, and k is the heat values relationship, 1.4 for nitrogen.Then solving for 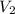 the volumetric flow at the exit of the diffuser:
the volumetric flow at the exit of the diffuser: ![V_2=V_1*\frac{1}{\sqrt[k]{\frac{P_2}{P_1}}}=\frac{1.35\frac{m^3}{s}}{\sqrt[1.4]{\frac{0.9bar}{0.656bar} }}=1.080\frac{m^3}{s}](/tpl/images/0425/7077/99ad6.png) .Knowing that
.Knowing that 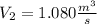 , it is possible to calculate the area at the exit of the diffuser, using the relationship presented in step 4, and solving for the required parameter:
, it is possible to calculate the area at the exit of the diffuser, using the relationship presented in step 4, and solving for the required parameter: 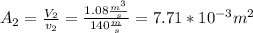 .To determine the rate of entropy production in the diffuser, it is required to do a second law balance (entropy balance) in the control volume of the device. This balance is:
.To determine the rate of entropy production in the diffuser, it is required to do a second law balance (entropy balance) in the control volume of the device. This balance is: 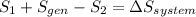 , where:
, where: 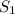 and
and 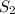 are the entropy of the stream entering and leaving the control volume respectively,
are the entropy of the stream entering and leaving the control volume respectively, 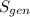 is the rate of entropy production and,
is the rate of entropy production and, 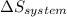 is the change of entropy of the system.If the diffuser is operating at stable state is assumed then
is the change of entropy of the system.If the diffuser is operating at stable state is assumed then 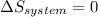 . Applying the entropy balance and solving the rate of entropy generation:
. Applying the entropy balance and solving the rate of entropy generation: 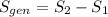 .Finally, it was assume that the process is isentropic, it is:
.Finally, it was assume that the process is isentropic, it is: 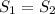 , then
, then 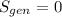 .
.

