Chemistry, 19.12.2019 07:31 datzmypupppup
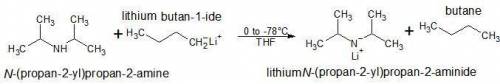
Lithium diisopropylamide is a strong, nonnucleophilic base. it is often freshly prepared by treating a certain reactant with n-butyllithium (n-buli). draw the starting material and draw the product (lithium diisopropylamide). include any charges, but you do not need to draw electron pairs.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

You know the right answer?
Lithium diisopropylamide is a strong, nonnucleophilic base. it is often freshly prepared by treating...
Questions


Chemistry, 05.10.2019 09:01


Mathematics, 05.10.2019 09:01

Chemistry, 05.10.2019 09:01

Mathematics, 05.10.2019 09:01


Social Studies, 05.10.2019 09:01

Mathematics, 05.10.2019 09:01

Social Studies, 05.10.2019 09:01

Chemistry, 05.10.2019 09:01

Chemistry, 05.10.2019 09:01

Mathematics, 05.10.2019 09:01



Mathematics, 05.10.2019 09:01