
Chemistry, 19.12.2019 20:31 harshakayla02
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g) + nh3(g) nh4cl(s) based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of nh4cl(s) is kj/mol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2



Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
Ascientist measures the standard enthalpy change for the following reaction to be -190.0 kj : hcl(g...
Questions

Mathematics, 17.03.2020 23:11




Physics, 17.03.2020 23:11

Mathematics, 17.03.2020 23:12


History, 17.03.2020 23:12

Mathematics, 17.03.2020 23:12



Mathematics, 17.03.2020 23:12

Mathematics, 17.03.2020 23:12


Mathematics, 17.03.2020 23:12


History, 17.03.2020 23:12


English, 17.03.2020 23:12

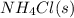 is -328.4 kJ/mol
is -328.4 kJ/mol![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(products)}]-\sum [n\times \Delta H_f_{(reactants)}]](/tpl/images/0426/4202/192b9.png)
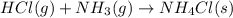
![\Delta H_{rxn}=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times \Delta H_f_{(NH_3(g))})+(1\times \Delta H_f_{(HCl(g))})]](/tpl/images/0426/4202/589ac.png)
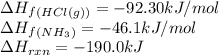
![-190.0=[(1\times \Delta H_f_{(NH_4Cl(s))})]-[(1\times (-46.1))+(1\times (-92.30))]\\\\\Delta H_f_{(NH_4Cl(s))}=-328.4kJ/mol](/tpl/images/0426/4202/9648b.png)


