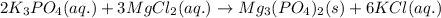Chemistry, 19.12.2019 20:31 raylynnreece5052
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium phosphate product was formed. consider the other product and its phase, and then write the balanced molecular equation for this precipitation reaction. express your answer as a chemical equation including phases.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Aqueous potassium phosphate was mixed with aqueous magnesium chloride, and a crystallized magnesium...
Questions


Mathematics, 01.03.2021 17:40

Business, 01.03.2021 17:40



Arts, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

English, 01.03.2021 17:40


Mathematics, 01.03.2021 17:40


Chemistry, 01.03.2021 17:40


History, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40

Social Studies, 01.03.2021 17:40

Mathematics, 01.03.2021 17:40


Mathematics, 01.03.2021 17:40