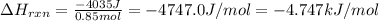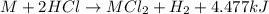Chemistry, 19.12.2019 22:31 keilyjaramillo2870
Consider the reaction: m + 2hcl → mcl2 + h2 when 0.85 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 4035 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? ) it is possible that your answer could be either positive or negative. if it is negative, you must include the "minus" sign. enter your answer as a decimal number.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

You know the right answer?
Consider the reaction: m + 2hcl → mcl2 + h2 when 0.85 mol of the metal, m, reacted with an aqueous...
Questions


Mathematics, 27.03.2021 16:30



Mathematics, 27.03.2021 16:40

English, 27.03.2021 16:40




Mathematics, 27.03.2021 16:40

Business, 27.03.2021 16:40



Biology, 27.03.2021 16:40






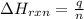
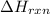 = enthalpy change of the reaction
= enthalpy change of the reaction