Chemistry, 19.12.2019 22:31 mostman077
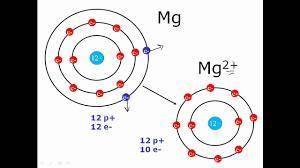
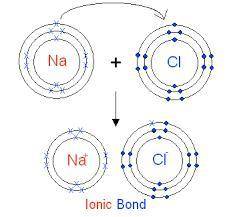
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the octet rule state that atoms form ions in an effort to acquire valence electron configuration impermanence.
b) both the duet and octet rules reveal that atoms gain or lose electrons forming ions to achieve a more stable electron configuration.
c) ion formation only occur if the atom have a full duet electron configuration or full octet electron configuration of valence electrons.
d) formation of immutable noble-gas configuration ions results from atoms with ambiguous duet or octet configurations of valence electrons.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the o...
a) the duet rule or the o...
Questions

Spanish, 30.10.2020 18:30



Mathematics, 30.10.2020 18:30


English, 30.10.2020 18:30


Social Studies, 30.10.2020 18:30

Mathematics, 30.10.2020 18:30


Mathematics, 30.10.2020 18:30

Mathematics, 30.10.2020 18:30

Mathematics, 30.10.2020 18:30


Engineering, 30.10.2020 18:30


Mathematics, 30.10.2020 18:30

Mathematics, 30.10.2020 18:30

History, 30.10.2020 18:30