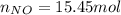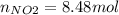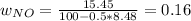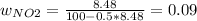Chemistry, 20.12.2019 00:31 ashleypere99
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reactions come to equilibrium after combustion in an internal-combustion engine at 2000 k and 200 bar, estimate the mole fractions of no and no2 present for mole fractions of nitrogen and oxygen in the combustion products of 0.70 and 0.05.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

You know the right answer?
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reaction...
Questions

Mathematics, 05.05.2021 17:10

Chemistry, 05.05.2021 17:10




Mathematics, 05.05.2021 17:10

History, 05.05.2021 17:10

Advanced Placement (AP), 05.05.2021 17:10

History, 05.05.2021 17:10

Mathematics, 05.05.2021 17:10

Social Studies, 05.05.2021 17:10

Mathematics, 05.05.2021 17:10


Physics, 05.05.2021 17:10

Mathematics, 05.05.2021 17:10



Mathematics, 05.05.2021 17:10

History, 05.05.2021 17:10

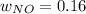
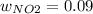
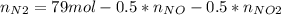
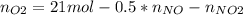
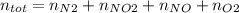
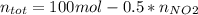
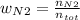
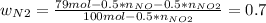
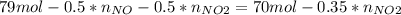
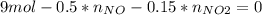 (1)
(1)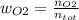
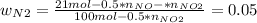
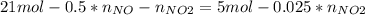
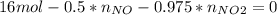 (2)
(2)