
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd(s) 2 ag(s) + cd2+ (a) voltage increases. (b) voltage decreases but remains > zero. (c) voltage becomes zero and remains at zero. (d) no change in voltage occurs. (e) direction of voltage change cannot be predicted without additional information. which of the above occurs for each of the following circumstances? 14. a 50-milliliter sample of a 2-molar cd(no3)2 solution is added to the left beaker. 15. the silver electrode is made larger. 16. the salt bridge is replaced by a platinum wire. 17. current is allowed to flow for 5 minutes.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1
You know the right answer?
The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2ag+ + cd...
Questions

English, 27.04.2021 01:00



Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


Biology, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00


Social Studies, 27.04.2021 01:00

History, 27.04.2021 01:00

English, 27.04.2021 01:00

Social Studies, 27.04.2021 01:00


English, 27.04.2021 01:00



Social Studies, 27.04.2021 01:00

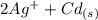 ⇒
⇒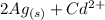
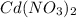 solution is added to the left beaker.
solution is added to the left beaker. 

