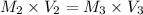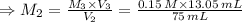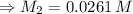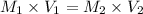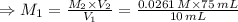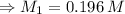Chemistry, 20.12.2019 02:31 halimomohamed
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer flask. an excess of ki solution was added. indicator was added and the solution was diluted with h2o to a total volume of 75 ml. for rxn 2, the solution from rxn 1 was titrated with 0.15 m na2s2o3. the equivalence point of the titration was reached when 13.05 ml of na2s2o3 had been added. what is the molar concentration of cu2+ in the original 10.0 ml solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer fla...
Questions

Mathematics, 15.01.2021 21:50

Mathematics, 15.01.2021 21:50


Mathematics, 15.01.2021 21:50

Mathematics, 15.01.2021 21:50

Mathematics, 15.01.2021 21:50

English, 15.01.2021 21:50








Mathematics, 15.01.2021 21:50

History, 15.01.2021 21:50


Mathematics, 15.01.2021 21:50


Biology, 15.01.2021 21:50