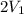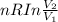Chemistry, 20.12.2019 03:31 arifkarimi9214
Calculate the change in the entropy of the system and also the change in the entropy of the surroundings, and the resulting total change in entropy, when a sample of nitrogen gas of mass 14 g at 298 k and 1.00 bar doubles its volume in (a) an isothermal reversible expansion, (b) an isothermal irreversible expansion against pext = 0, and (c) an adiabatic reversible expansion.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
Calculate the change in the entropy of the system and also the change in the entropy of the surround...
Questions


Mathematics, 30.03.2021 05:00


Social Studies, 30.03.2021 05:00


Chemistry, 30.03.2021 05:00

Mathematics, 30.03.2021 05:00



Mathematics, 30.03.2021 05:00

Mathematics, 30.03.2021 05:00


Mathematics, 30.03.2021 05:00



Computers and Technology, 30.03.2021 05:00




History, 30.03.2021 05:00

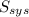 = 2.881 J/K; Δ
= 2.881 J/K; Δ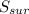 = -2.881 J/K; total change in entropy = 0
= -2.881 J/K; total change in entropy = 0 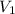
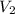 =
=