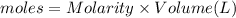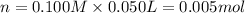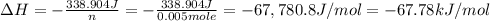Chemistry, 20.12.2019 03:31 cancerbaby209
When 50.0 ml of 0.100 m agno3 is mixed with 50.0 ml of 0.100 m hcl in a coffee cup calorimeter, the temperature increases from 23.40 °c to 24.21°c. if the specific heat of the solution is 4.184 j/°c·g and its density is 1.00 g/ml, assuming that the calorimeter undergoes no heat change, calculate the enthalpy change for the following reaction in units of kj/mol agno3: agno3(aq) + hcl(aq) à agcl(s) + hno3(aq)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
When 50.0 ml of 0.100 m agno3 is mixed with 50.0 ml of 0.100 m hcl in a coffee cup calorimeter, the...
Questions



Mathematics, 04.01.2020 08:31

Biology, 04.01.2020 08:31





English, 04.01.2020 08:31

Social Studies, 04.01.2020 08:31



Social Studies, 04.01.2020 08:31

English, 04.01.2020 08:31



Mathematics, 04.01.2020 08:31

History, 04.01.2020 08:31

Health, 04.01.2020 08:31

Biology, 04.01.2020 08:31

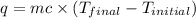
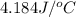
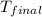 = final temperature =
= final temperature = 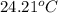
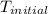 = initial temperature =
= initial temperature = 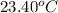
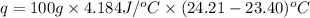
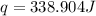
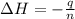
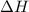 = enthalpy change = ?
= enthalpy change = ?