
Chemistry, 20.12.2019 06:31 michaellangley
Achemist must prepare of sodium hydroxide solution with a ph of at . he will do this in three steps: fill a volumetric flask about halfway with distilled water. weigh out a small amount of solid sodium hydroxide and add it to the flask. fill the flask to the mark with distilled water. calculate the mass of sodium hydroxide that the chemist must weigh out in the second step.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Achemist must prepare of sodium hydroxide solution with a ph of at . he will do this in three steps:...
Questions

Biology, 22.04.2020 20:47


Biology, 22.04.2020 20:47


Social Studies, 22.04.2020 20:47



Biology, 22.04.2020 20:47


Mathematics, 22.04.2020 20:47


Computers and Technology, 22.04.2020 20:47




Mathematics, 22.04.2020 20:47

Mathematics, 22.04.2020 20:47

Mathematics, 22.04.2020 20:47

Social Studies, 22.04.2020 20:47

Spanish, 22.04.2020 20:47

![pH = -log[H_3O^+]](/tpl/images/0427/4560/89cf7.png) .NaOH is a strong base, as it's a hydroxide formed with a group 1A metal, so it dissociates fully in water by the equation:
.NaOH is a strong base, as it's a hydroxide formed with a group 1A metal, so it dissociates fully in water by the equation: 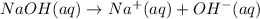 .From the equation above, using stoichiometry we can tell that the molarity of hydroxide is equal to the molarity of NaOH:
.From the equation above, using stoichiometry we can tell that the molarity of hydroxide is equal to the molarity of NaOH: ![[NaOH] = [OH^-]](/tpl/images/0427/4560/b5cb4.png) .Concentration of hydroxide is then equal to the ratio of moles of NaOH and the volume of the given solution. Moles themselves are equal to mass over molar mass, so we obtain:
.Concentration of hydroxide is then equal to the ratio of moles of NaOH and the volume of the given solution. Moles themselves are equal to mass over molar mass, so we obtain: ![[OH^-] = [NaOH] = \frac{n_{NaOH}}{V} = \frac{m_{NaOH}}{M_{NaOH}V}](/tpl/images/0427/4560/2bd04.png) .We also know that
.We also know that ![pOH = 14.00 - pH = -log[NaOH]](/tpl/images/0427/4560/e6921.png) . Take the antilog of both sides:
. Take the antilog of both sides: ![10^{-pOH} = 10^{pH - 14.00} = [NaOH] = \frac{m_{NaOH}}{M_{NaOH}V}](/tpl/images/0427/4560/d48cb.png) .Solve for the mass of NaOH:
.Solve for the mass of NaOH: 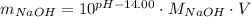 .
.


