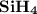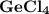1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
use the octet rule to predict the formula of the compound that would form between silicon and hydrogen , if the molecule contains only one silicon atom and only single bonds are formed.
formula:
2. the element germanium would be expected to covalent bond(s) in order to obey the octet rule. use the octet rule to predict the formula of the compound that would form between germanium and chlorine , if the molecule contains only one germanium atom and only single bonds are formed.
formula:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
1.the element silicon would be expected to covalent bond(s) in order to obey the octet rule.
<...
<...
Questions

Computers and Technology, 19.11.2020 07:50




Physics, 19.11.2020 07:50

Chemistry, 19.11.2020 07:50

Mathematics, 19.11.2020 07:50





Mathematics, 19.11.2020 07:50




Medicine, 19.11.2020 07:50

Mathematics, 19.11.2020 07:50

Mathematics, 19.11.2020 07:50

Arts, 19.11.2020 07:50

Chemistry, 19.11.2020 07:50