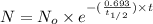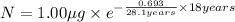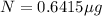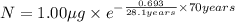Chemistry, 20.12.2019 06:31 bryan12376
One of the hazards of nuclear explosions is the generation of 90sr andits subsequent incorporation in place of calcium in bones. this nuclide emitsβrays of energy 0.55 mev, and has a half-life of 28.1 y. suppose 1.00 μg wasabsorbed by a newly born child. how much will remain after (a) 18 y, (b) 70 yif none is lost metabolically?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
One of the hazards of nuclear explosions is the generation of 90sr andits subsequent incorporation i...
Questions





History, 22.06.2019 19:00

Business, 22.06.2019 19:00

History, 22.06.2019 19:00



French, 22.06.2019 19:00


Chemistry, 22.06.2019 19:00



Mathematics, 22.06.2019 19:00

Mathematics, 22.06.2019 19:00




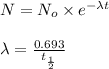
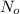 = initial mass of isotope
= initial mass of isotope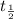 = half life of the isotope = 28.1 years
= half life of the isotope = 28.1 years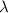 = rate constant
= rate constant