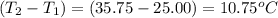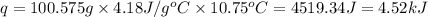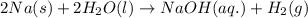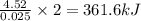Chemistry, 20.12.2019 18:31 King1Gates
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemical equation shown below.
when 0.0 25 mol of na is added to 100.00 g of water, the temperature of the resulting solution rises from 25.00°c to 35.75°c.
if the specific heat of the solution is 4.18 j/(g · °c), calculate δh for the reaction, as written.
2 na(s) + 2 h2o(l) → 2 naoh(aq) + h2(g) δh= ?

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to the chemica...
Questions













Mathematics, 14.09.2019 10:30







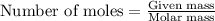
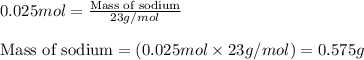
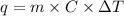
 = change in temperature =
= change in temperature =