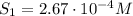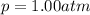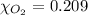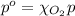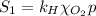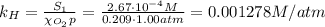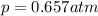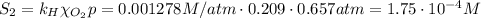The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the solubility of o2 from the air is 2.67 ✕ 10-4 m at sea level and 25°c, what is the solubility of o2 at an elevation of 12,000 ft where the atmospheric pressure is 0.657 atm? assume the temperature is 25°c, and that the mole fraction of o2 in air is 0.209 at both 12,000 ft and at sea level.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
The solubility of oxygen in lakes high in the rocky mountains is affected by the altitude. if the so...
Questions

Spanish, 16.04.2021 17:50


Computers and Technology, 16.04.2021 17:50

Mathematics, 16.04.2021 17:50


Social Studies, 16.04.2021 17:50

Physics, 16.04.2021 17:50

Mathematics, 16.04.2021 17:50



Mathematics, 16.04.2021 17:50



English, 16.04.2021 17:50



Biology, 16.04.2021 17:50


Mathematics, 16.04.2021 17:50

Mathematics, 16.04.2021 17:50

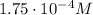
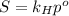
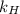 is Henry's law constant.
is Henry's law constant.